High ($100 $150) To do this science fair project, you will need to electrolyze water into hydrogen and oxygen. Hydrogen is flammable, so keep the fuel cell and hydrogen storage tank away from sparks. Michelle Maranowski, PhD, Science Buddies Investigate how a fuel cell works and determine its efficiency.
What is a hydrogen car and how does it work?
Basic Principles of Hydrogen Fuel Cells. . A hydrogen fuel cell is an electrochemical device that converts the chemical energy stored in hydrogen and oxygen into electrical energy. The process involves combining hydrogen and oxygen in the presence of a catalyst to produce electricity, water, and heat. .

Source Image: freepik.com
Download Image
Figure 9.6. Common combinations of fuel and electrolytes. 9.4.1 Alkaline Electrolytes Hydrogen-oxygen fuel cells with alkaline electrolytes (generally, KOH) use OH − as the current-carrying ion. Because the ion contains oxygen, water is formed at the anode. The KOH in the electrolyte dissociates: (9.6)
Source Image: chegg.com
Download Image
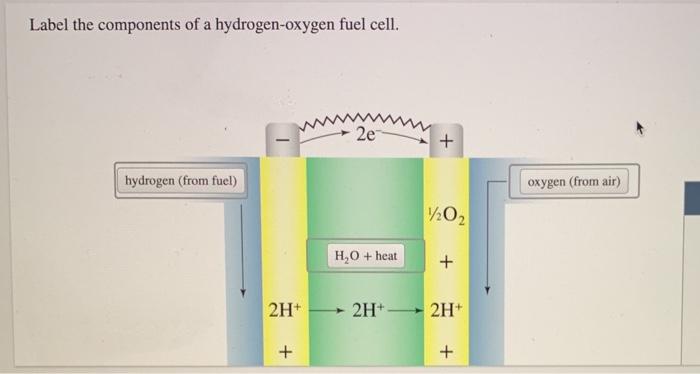
SOLVED: Labcl the components of a hydrogen-oxygen fuel cell, hydrogen (H2) oxygen (O2) anode (negative electrode) cathode (positive electrode) electrolyte (conducting medium) Fuel Cell Basics. A fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. Fuel cells are used today in a range of applications, from providing power to homes and businesses, keeping critical facilities like hospitals, grocery stores, and data centers up and
Source Image: alamy.com
Download Image
Label The Components Of A Hydrogen Oxygen Fuel Cell
Fuel Cell Basics. A fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. Fuel cells are used today in a range of applications, from providing power to homes and businesses, keeping critical facilities like hospitals, grocery stores, and data centers up and Nov 13, 2022The most well-known primary battery has long been the common “dry cell” that is widely used to power flashlights and similar devices. The modern dry cell is based on the one invented by Georges Leclanché in 1866. The electrode reactions are. Zn → Zn2+ + 2e- (16.6.3) (16.6.3) Z n → Z n 2 + + 2 e -.
Hydrogen oxygen fuel cell hi-res stock photography and images – Alamy
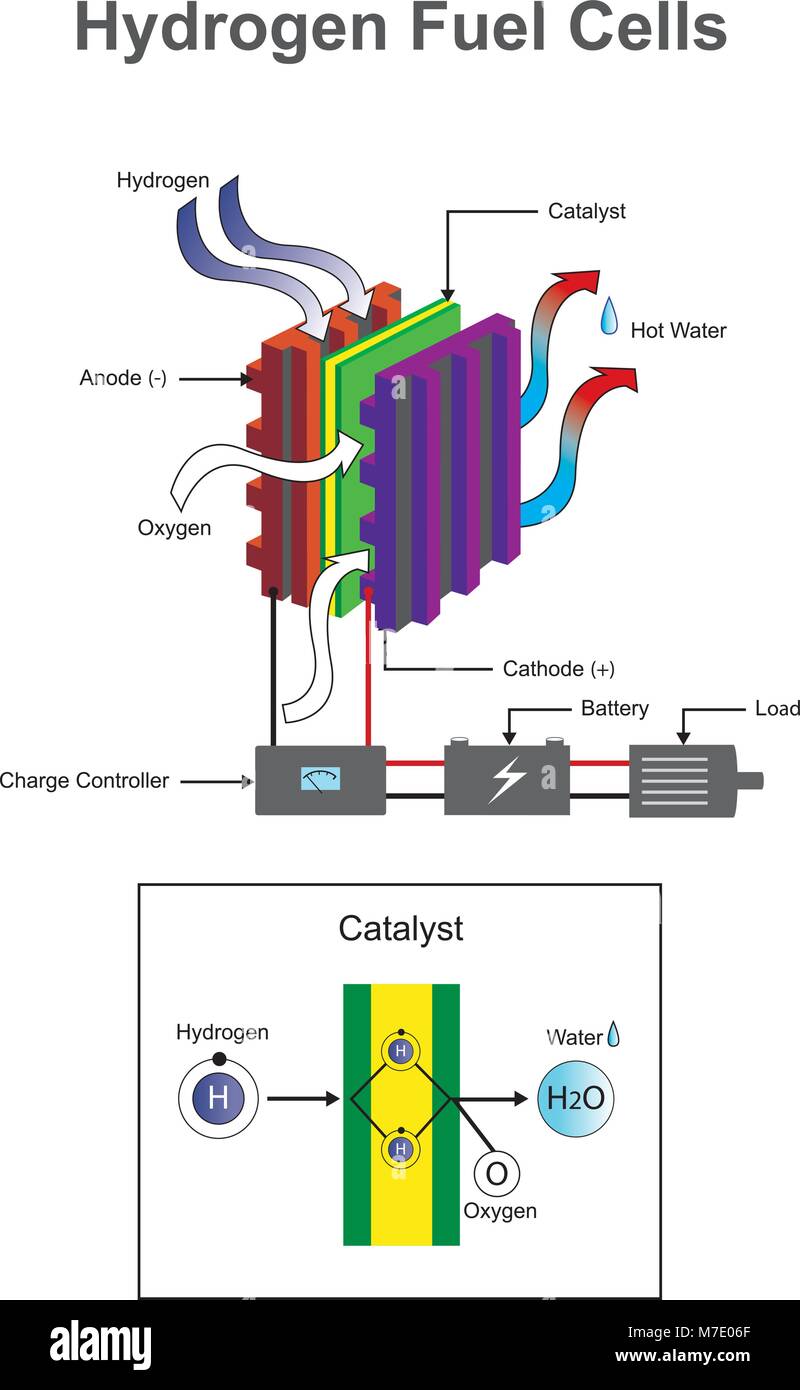
The polymer electrolyte membrane, or PEM (also called a proton exchange membrane)—a specially treated material that looks something like ordinary kitchen plastic wrap—conducts only positively charged ions and blocks the electrons. Hydrogen Production Process Photos and Images | Shutterstock

Source Image: shutterstock.com
Download Image
Hydrogen-Oxygen Reaction Lab – Activity – TeachEngineering The polymer electrolyte membrane, or PEM (also called a proton exchange membrane)—a specially treated material that looks something like ordinary kitchen plastic wrap—conducts only positively charged ions and blocks the electrons.
Source Image: teachengineering.org
Download Image
What is a hydrogen car and how does it work? High ($100 $150) To do this science fair project, you will need to electrolyze water into hydrogen and oxygen. Hydrogen is flammable, so keep the fuel cell and hydrogen storage tank away from sparks. Michelle Maranowski, PhD, Science Buddies Investigate how a fuel cell works and determine its efficiency.
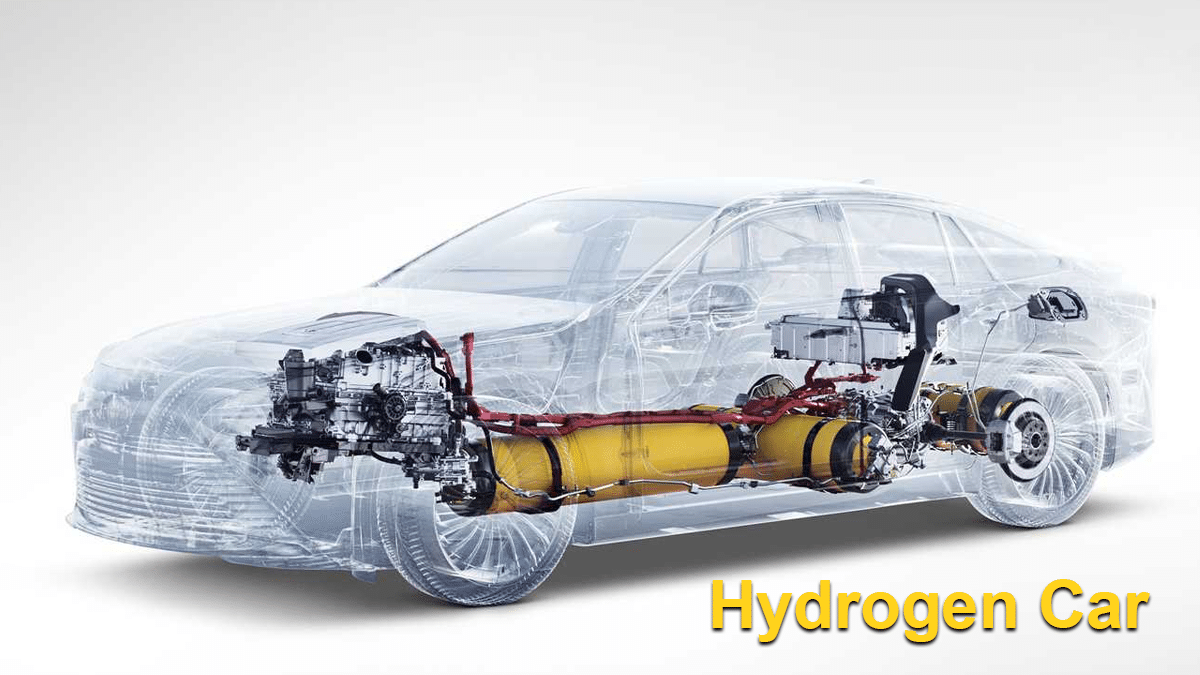
Source Image: blog.smeclabs.com
Download Image
SOLVED: Labcl the components of a hydrogen-oxygen fuel cell, hydrogen (H2) oxygen (O2) anode (negative electrode) cathode (positive electrode) electrolyte (conducting medium) Figure 9.6. Common combinations of fuel and electrolytes. 9.4.1 Alkaline Electrolytes Hydrogen-oxygen fuel cells with alkaline electrolytes (generally, KOH) use OH − as the current-carrying ion. Because the ion contains oxygen, water is formed at the anode. The KOH in the electrolyte dissociates: (9.6)
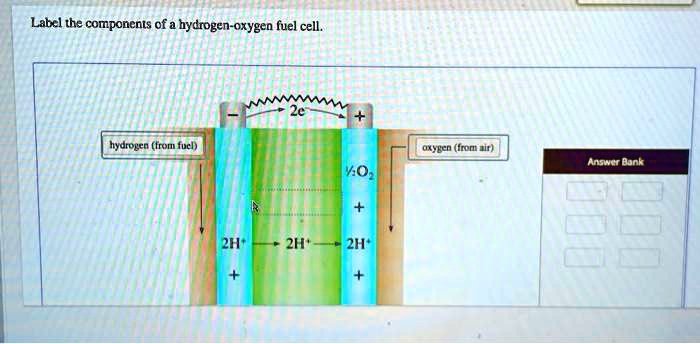
Source Image: numerade.com
Download Image
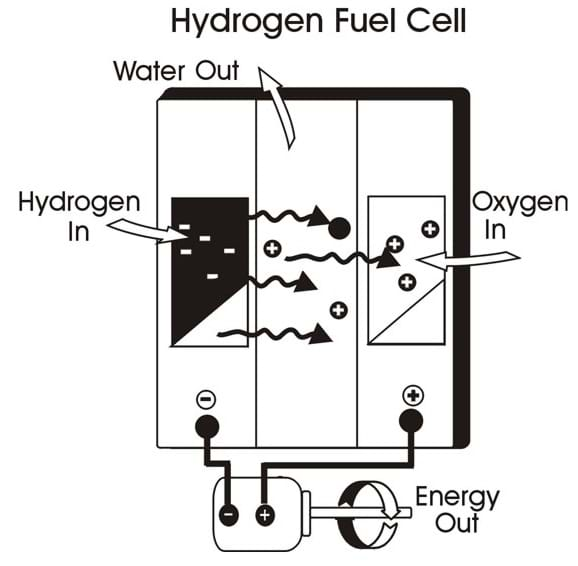
DIY Fuel Cell Science Kit – Arbor Scientific Power sources that can be used to electrolyze water into its constituent elements of hydrogen and oxygen are solar panels, battery packs, rechargeable batteries, or AC/DC adaptors. WARNING ! It is important that the voltage and current from electrical sources do not damage the fuel cell.

Source Image: arborsci.com
Download Image
Hydrogen oxygen fuel cell 21669326 Vector Art at Vecteezy Fuel Cell Basics. A fuel cell is a device that generates electricity through an electrochemical reaction, not combustion. In a fuel cell, hydrogen and oxygen are combined to generate electricity, heat, and water. Fuel cells are used today in a range of applications, from providing power to homes and businesses, keeping critical facilities like hospitals, grocery stores, and data centers up and
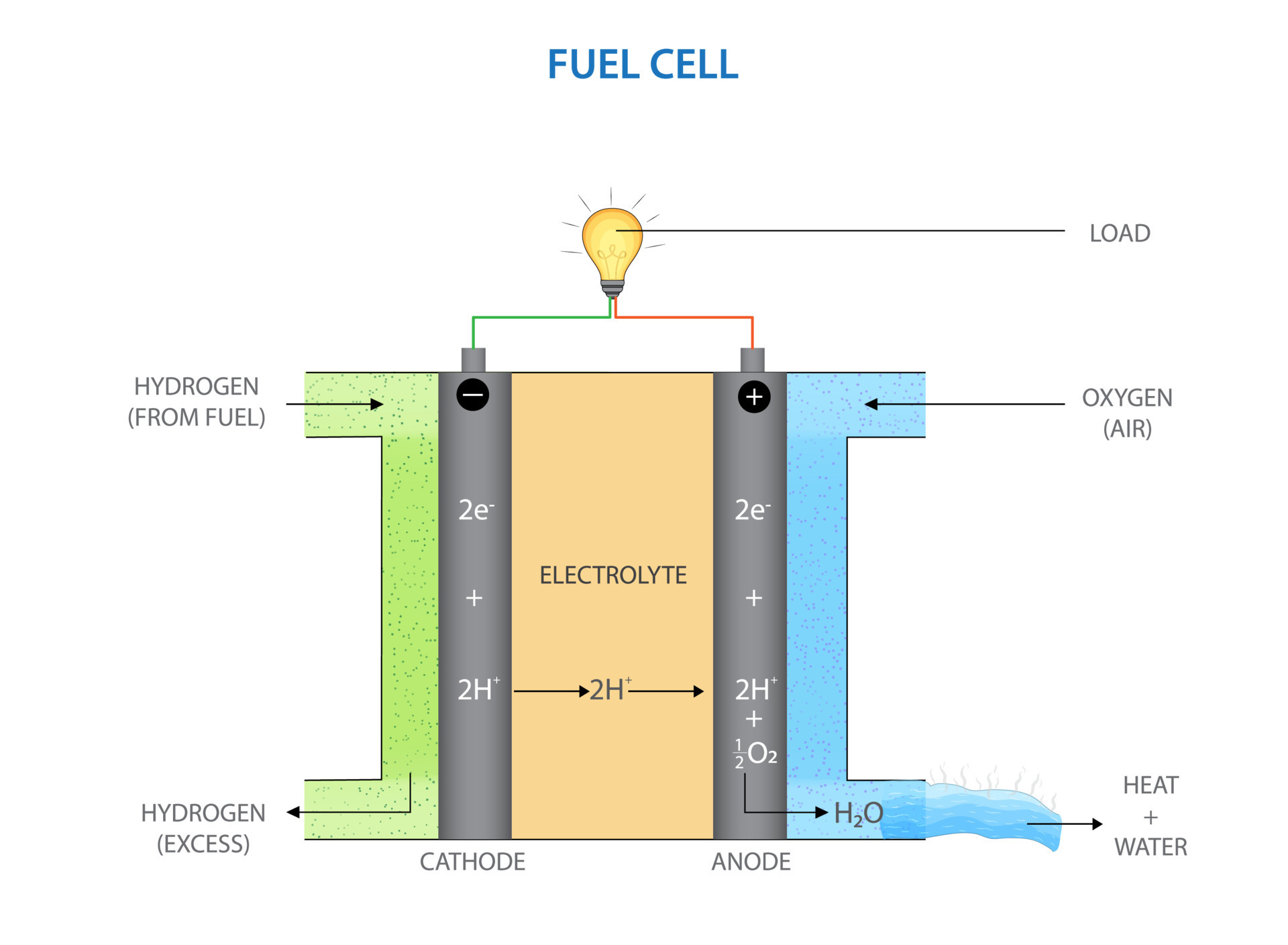
Source Image: vecteezy.com
Download Image
Stirling engine generator and fuel cell diagram Vector Image Nov 13, 2022The most well-known primary battery has long been the common “dry cell” that is widely used to power flashlights and similar devices. The modern dry cell is based on the one invented by Georges Leclanché in 1866. The electrode reactions are. Zn → Zn2+ + 2e- (16.6.3) (16.6.3) Z n → Z n 2 + + 2 e -.

Source Image: vectorstock.com
Download Image
Hydrogen-Oxygen Reaction Lab – Activity – TeachEngineering
Stirling engine generator and fuel cell diagram Vector Image Basic Principles of Hydrogen Fuel Cells. . A hydrogen fuel cell is an electrochemical device that converts the chemical energy stored in hydrogen and oxygen into electrical energy. The process involves combining hydrogen and oxygen in the presence of a catalyst to produce electricity, water, and heat. .
SOLVED: Labcl the components of a hydrogen-oxygen fuel cell, hydrogen (H2) oxygen (O2) anode (negative electrode) cathode (positive electrode) electrolyte (conducting medium) Hydrogen oxygen fuel cell 21669326 Vector Art at Vecteezy Power sources that can be used to electrolyze water into its constituent elements of hydrogen and oxygen are solar panels, battery packs, rechargeable batteries, or AC/DC adaptors. WARNING ! It is important that the voltage and current from electrical sources do not damage the fuel cell.